Evaluating Redosable Next-Generation Gene Delivery for Retinal Applications
Overview:
Stargardt Disease is an inherited macular dystrophy disease characterized by progressive vision loss. This condition is caused by mutations in the ABC4 gene, a large 6.8 kbp gene that plays a role in transporting waste products from the retina, leading to their accumulation and damage to photoreceptor cells. Despite the immense promise of gene therapy for treatment of this condition, its clinical translation is complicated by two major obstacles: the considerable size of the ABCA4 gene, which far exceeds the packaging capacity of conventional gene therapy vectors, and the challenge of efficient, non-invasive delivery of therapeutic DNA to the eye, particularly the posterior retina. Existing methods often face limitations due to numerous ocular barriers and the restricted packaging capacity, highlighting an unmet need for safe, redosable, and efficient solutions capable of transporting large gene constructs to specific retinal cell types.
Retinal Gene Delivery with Optimized Ministring DNA
Using a novel approach of minimally invasive and redosable linear covalently closed (LCC) DNA minivectors, known as “ministring DNA” (msDNA), these obstacles can be mitigated.
msDNA vectors are LCC DNA that contain the gene of interest and elements needed for its expression, without any of the prokaryotic sequences characteristic of conventional DNA vectors. A one-step in vivo prokaryotic platform was used to generate msDNA vectors carrying a replacement of the ABC4 gene (msDNA-ABC4) for delivery to rodent models. To maximize therapeutic efficacy, our constructs incorporated super sequences (SSeq), which are specifically utilized to enhance the nuclear translocation of the gene cassette within target cells. We also integrated robust promoters (CMV or CAG) to control the expression of the therapeutic gene (ABCA4) and a reporter gene (GFP) for both visualization and precise measurement of expression levels.
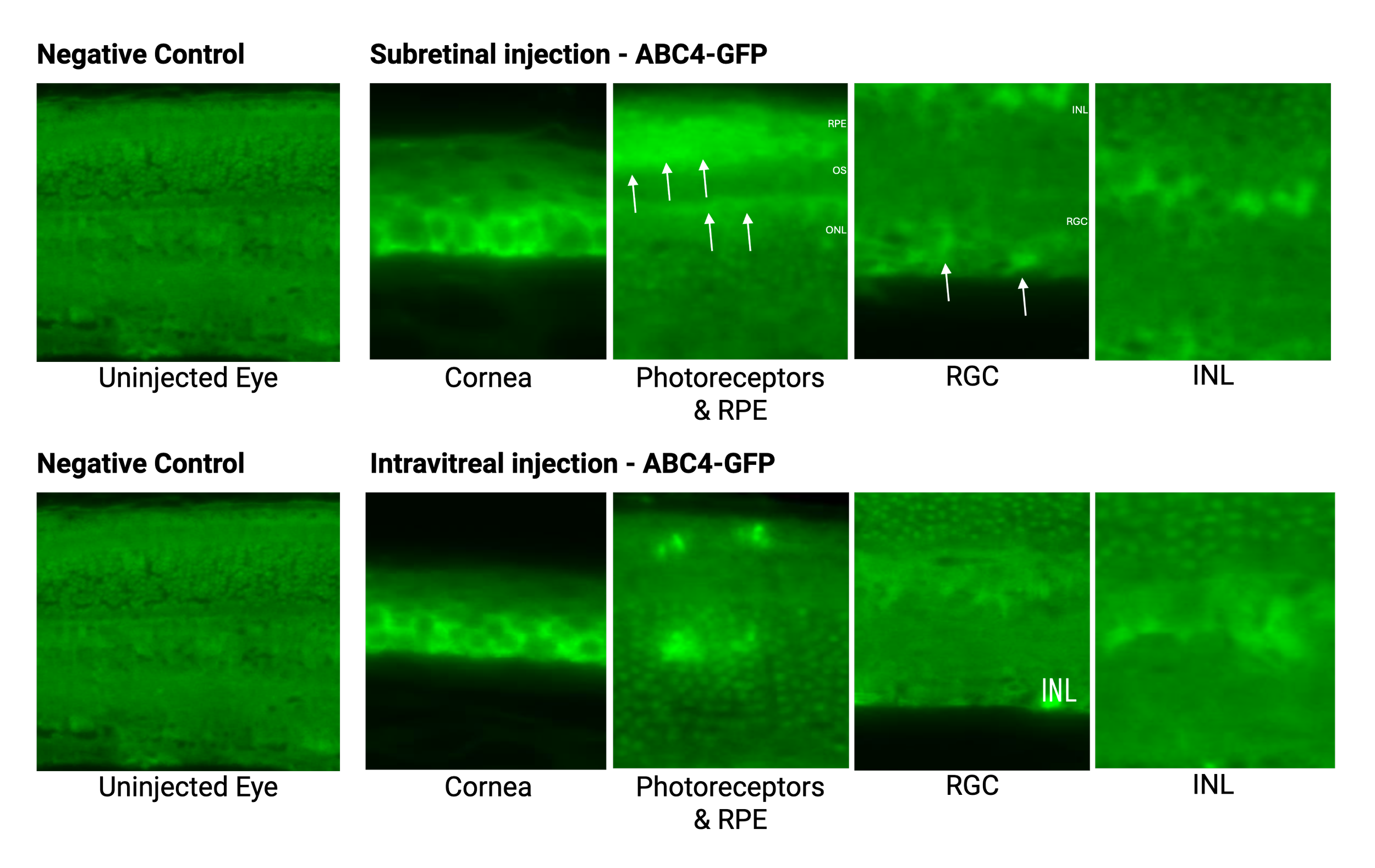
In vivo analyses detail the distribution and expression of msDNA-ABC4 in various retinal cell layers confirming their potential for therapeutic applications with sustained expression. We also explored the potential use of msDNA constructs for ocular gene therapy using less invasive routes of administration, including suprachoroidal injection (SupC) and intravitreal injection (IVT) injection, compared to the conventional but more invasive subretinal injection (SubR) route.
msDNA-ABC4 retinal distribution after injection in mice. The arrows highlight GFP expression in different regions of the eye. Subretinal injection: GFP expression in ocular tissue after 1 week. Intravitreal injection: GFP expression for 3 weeks after two IVT injections.
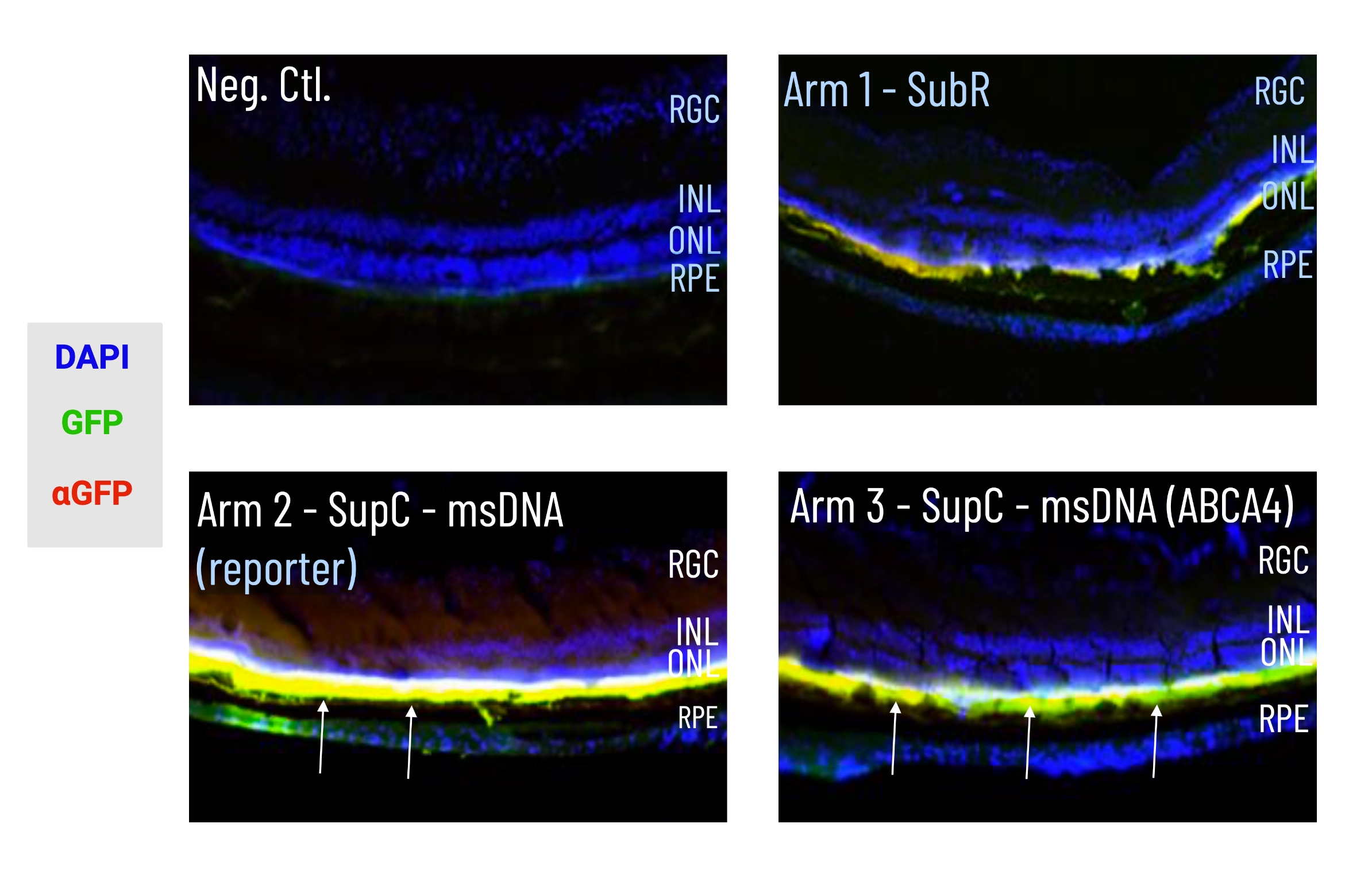
msDNA-ABC4 expression within photoreceptor layers and RPE after injection in mice. DAPI was used to stain the nuclear DNA of cells, the arrows indicate regions of GFP expression.
Further Investigating Retinal Gene Delivery with Phage-based Vectors
We continue to investigate potential avenues for ocular gene therapy, including using engineered M13-miniphagemid vectors for targeted delivery of the ABC4 msDNA vectors, as a application of the iPhAGE System. The iPhAGE platform will be used to generate single stranded DNA (ssDNA) and LCC DNA vectors with fusogenic and targeting properties towards photoreceptors to deliver the ABCA4 gene. Given the penetrative capacity of filamentous phage, iPhAGEs will be tested via typical routes of administration, but we will also explore development of this system for functional, non-invasive, and repeatable delivery as eye drops.
M13-miniphagemids are produced in an one-step in vitro system within E. coli using a specialized helper plasmid and DNA vector plasmid. The helper plasmid contains all the necessary elements for replication, transcription, and assembly of the M13 miniphagemid capsid, while the DNA vector plasmid encodes the gene of interest, in this case the ABCA4 gene. The capacity for multiple dosing and modifiability of the M13 miniphagemids may also offer flexibility and treatment personalization.
To optimize the iPhAGE system for this specific application, several modifications were made:
The helper plasmid provides the phage proteins necessary for miniphagemid assembly and cell specific targeting.
Modified for display of peptides are engineered to enhance retinal protection and membrane permeability, facilitating targeted and efficient delivery.
The precursor plasmid provides the gene cassette (including the gene of interest) that is packaged within the miniphagemid vector.
Modified to encode the therapeutic gene (ABCA4) and a reporter gene (GFP) for both visualization and precise measurement of expression levels.
Modified to encode robust promoters, CMV and CAG, to drive the expression of the gene cassette.
This platform has generated promising targeted in vitro data, demonstrating its capacity for precise gene delivery. The inherently high penetrative capacity of phage-based systems, combined with our novel minivector designs, suggests a promising path for non-invasive and redosable gene delivery directly to photoreceptors.
This technology holds significant potential for translating to the treatment of Stargardt Disease and other inherited retinopathies and macular degenerative disorders. Our work represents a crucial step towards developing a versatile and highly effective gene therapy for a broad spectrum of ocular diseases.
Publications:
Aghajanpour, S., Amiriara, H., Ebrahimnejad, P., Slavcev, R.A. (2025). Advancing ocular gene therapy: a machine learning approach to enhance delivery, uptake and gene expression. Drug Disocvery Today, 30:5
Key Researchers: